

The proton carries a positive electrical charge, that is equal in magnitude to the charge of the electron but opposite in sign.
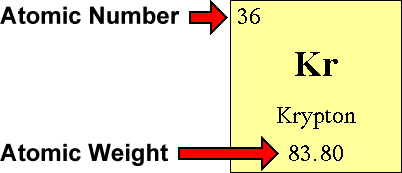
There is another unit, called an electron volt (eV), that scientists use when talking about small things like protons, neutrons and electrons. The mass of a proton is 1.6726×10 24 g, or about 1836 times the mass of an electron.
The gram (alternative spelling: gramme SI unit symbol: g) is a metric system unit of mass. Recent observations with VLT/UVES support the view that the neutron stars in high-mass X-ray binaries display a. Always check the results rounding errors may occur. Find the de-Broglie wavelength of the neutron. The kinetic energy of a neutron of kg mass is 0.04 eV.
Mass of a eutron how to#
While relative masses are nice if you want to compare protons, neutrons and electrons to one another, it doesn't tell you what the actual masses of these particles are. How to convert Neutron Mass to Grams (n0 to g) 1 n0 1.6749286E-24 g. In order to have the same wavelength for the electron (mass ) and the neutron (mass ) their velocities should be in the ratio (electron veloctiy/neutron veloctity) :- 14157486. Below are the relations for neutron energy E given in meV (i.e. Said another way, protons are only about 99.86% as massive as neutrons while electrons are only about 0.054% as massive as neutrons. Let m1.6749 10-27 kg be the neutron mass, v its velocity and h Plancks constant. If we assume that a neutron has a mass of 1, then the relative masses are: Protons and neutrons have nearly the same mass while electrons are much less massive. Exhibit a positive charge Found in the nucleus Abbreviated p+ Mass. What are the exact relative masses of protons, neutrons and electrons? An atom is made of three subatomic particles: protons, neutrons, and electrons.


 0 kommentar(er)
0 kommentar(er)
